Teplizumab is a medicine, not too long ago authorized in the USA, to delay the onset of stage 3 sort 1 diabetes mellitus in adults and kids over the age of 8 years. It’s not but out there in Australia.
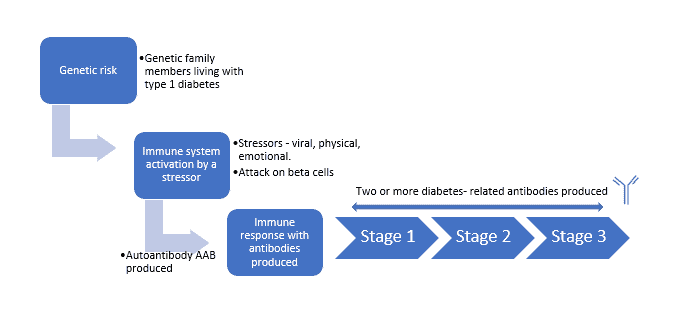
Type 1 diabetes mellitus is a mixture of genetic susceptibility and a stressor that begins an autoimmune cascade that destroys the beta cells (insulin-producing) within the pancreas.
- Individuals with a first-degree relative with sort 1 diabetes have a 1 in 20 danger of sort 1 diabetes. The final inhabitants has a danger of 1 in 300.
- The autoimmune cascade includes one’s immune system not recognising its personal cells will be triggered by a viral an infection or chemical, bodily, or emotional stress.
- When our physique detects a overseas physique, our immune system produces particular neutralising proteins known as antibodies. When these are directed towards our personal physique they’re known as autoantibodies. These autoantibodies will be discovered with blood evaluation.
- 5 autoantibodies are markers of beta cell autoimmunity in sort 1 diabetes: islet cell antibodies (ICA), towards cytoplasmic proteins within the beta cell, antibodies to glutamic acid decarboxylase 65 (GAD), insulin autoantibodies (IAA), Insulinoma-associated antigen 2 autoantibody (IA-2A), and Zinc transporter 8 autoantibody (ZnT8A).
- Stage 1 of sort 1 diabetes happens when an individual has two or extra islet antibodies. At this stage, blood glucose ranges are usually not raised.
- Stage 2 happens when there are a number of islet antibodies, raised blood glucose, however no signs.
- At stage 3 the individual has raised blood glucose and is symptomatic.
- Stage 4 is long-standing sort 1 diabetes.
- Most youngsters susceptible to sort 1 diabetes with a number of islet antibodies progress to diabetes throughout the subsequent 15 years, in comparison with about 10% who’ve a single islet antibody.
Teplizumab, model title Tzield, is a (monoclonal antibody) treatment that has been authorized to be used by the Meals and Drug Administration (FDA) in the USA. Nonetheless, it’s but to be authorized in Australia by our Therapeutic Items Administration (TGA).
Tzield binds to particular immune system cells and delays development to stage 3 sort 1 diabetes. As well as, Tzield could deactivate the immune cells that assault insulin-producing cells whereas rising the proportion of cells that reasonable the immune response. Tzield is run by intravenous infusion as soon as day by day for 14 consecutive days. For many who reply to Tzield, it delays sort 1 diabetes stage 3 onset by roughly two years. Observe-up research revealed that decline in beta cell perform was diminished for as much as seven years.
Precautions to be used embrace:
- Administering all age-appropriate vaccinations earlier than beginning Teplizumab.
- Reside-attenuated vaccines are usually not advisable inside eight weeks earlier than Teplizumab therapy, throughout therapy, or as much as 52 weeks after therapy. The vaccine response could also be affected after this time.
- Inactivated or mRNA vaccines are usually not advisable inside two weeks earlier than Teplizumab therapy, throughout therapy, or six weeks after completion of therapy.
- Adversarial results could embrace lymphopenia (73%), rash (36%), leukopenia (21%), headache (11%), and critical infections (9%). Much less generally it causes nausea, diarrhoea, runny nostril and sore throat, allergic response and alter in liver enzymes.
- Teplizumab can’t be utilized in being pregnant and has not been studied in kids below the age of 8 years.
The medical research for Teplizumab had been carried out by way of Type 1 Diabetes TrialNet, a global community of educational establishments, endocrinologists, physicians, scientists and healthcare groups on the forefront of sort 1 diabetes analysis. Trials for Tzield are closed; nevertheless, different trials are ongoing. In collaboration with The Royal Melbourne Hospital, Walter and Eliza Corridor Institute of medical analysis is a kind 1 diabetes TrialNet worldwide medical centre. (Type 1 Diabetes TrialNet, n.d.)
Why isn’t Teplizumab already out there in Australia?
Like different international locations, Australia has a security authority for treatment use, the Therapeutic Items Administration (TGA). The corporate that researched, developed and produced Teplizumab, Provention Bio, is predicated in the USA. FDA approval was granted late final yr after over three many years of analysis, medical trials and improvement. The following step is for the corporate to request approval from the TGA. When granted, the fee to the person should be very excessive until the Pharmaceutical Advantages Scheme subsidises it.
Who will profit from Teplizumab?
Teplizumab goals to delay the onset of stage 3 sort 1 diabetes in adults and paediatric sufferers aged eight years and older with stage 2 sort 1 diabetes. As soon as blood glucose ranges are symptomatic, typically 90% of beta cells have been destroyed, and Teplizumab might be ineffective.
As stage 2 sort 1 diabetes isn’t symptomatic, it is going to be these with a member of the family dwelling with sort 1 diabetes who will be genetically screened that will profit. Nonetheless, common screening will choose up phases 1 and a couple of of sort 1 diabetes.
Why would Teplizumab be used if it doesn’t stop sort 1 diabetes?
The danger of doable long-term results of dwelling with sort 1 diabetes will increase with the period of the situation. The individual has longer to dwell freed from insulin injections and glucose monitoring.
What’s prone to be the price of Tzield?
In line with estimates from Provention Bio, every 14-day therapy will price US$200,000. This price in the USA is prone to be inexpensive with private medical health insurance.
The choice to make use of this treatment sooner or later will weigh up the advantages towards dangers and prices. Nonetheless, this new treatment opens the door to hope for future improvement of remedies that will stop and, maybe, with cell substitute therapies, deal with sort 1 diabetes.
Donna Itzstein Pharmacist, Credentialled Diabetes Educator
Ana Luisa Perdigoto, P. P.-H. (2019). Therapy of sort 1 diabetes with Teplizumab: medical and immunological follow-up after 7 years from analysis. Diabetologia, 62, 655-664. doi:https://doi.org/10.1007/s00125-018-4786-9
Couper, J. H. (2018). ISPAD Scientific Follow Consensus Pointers 2018: Phases of sort 1 diabetes in kids and adolescents. Pediatric Diabetes, 19(27), 20-17. doi:https://doi.org/10.1111/pedi.12734
Kevan C. Herold, M. B. (2019, August 15). An Anti-CD3 Antibody, Teplizumab, in Kinfolk at Threat. The New England Journal of Medication, 381(7), 603-613.
Provention Bio, Inc. (2022, November). Tzield: Prescribing data. Retrieved January fifth, 2023, from Federal Drug Administration, https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761183s000lbl.pdf
Type 1 Diabetes TrialNet. (n.d.). Retrieved January fifth, 2023, from Type 1 Diabetes TrialNet: https://trialnet.org/